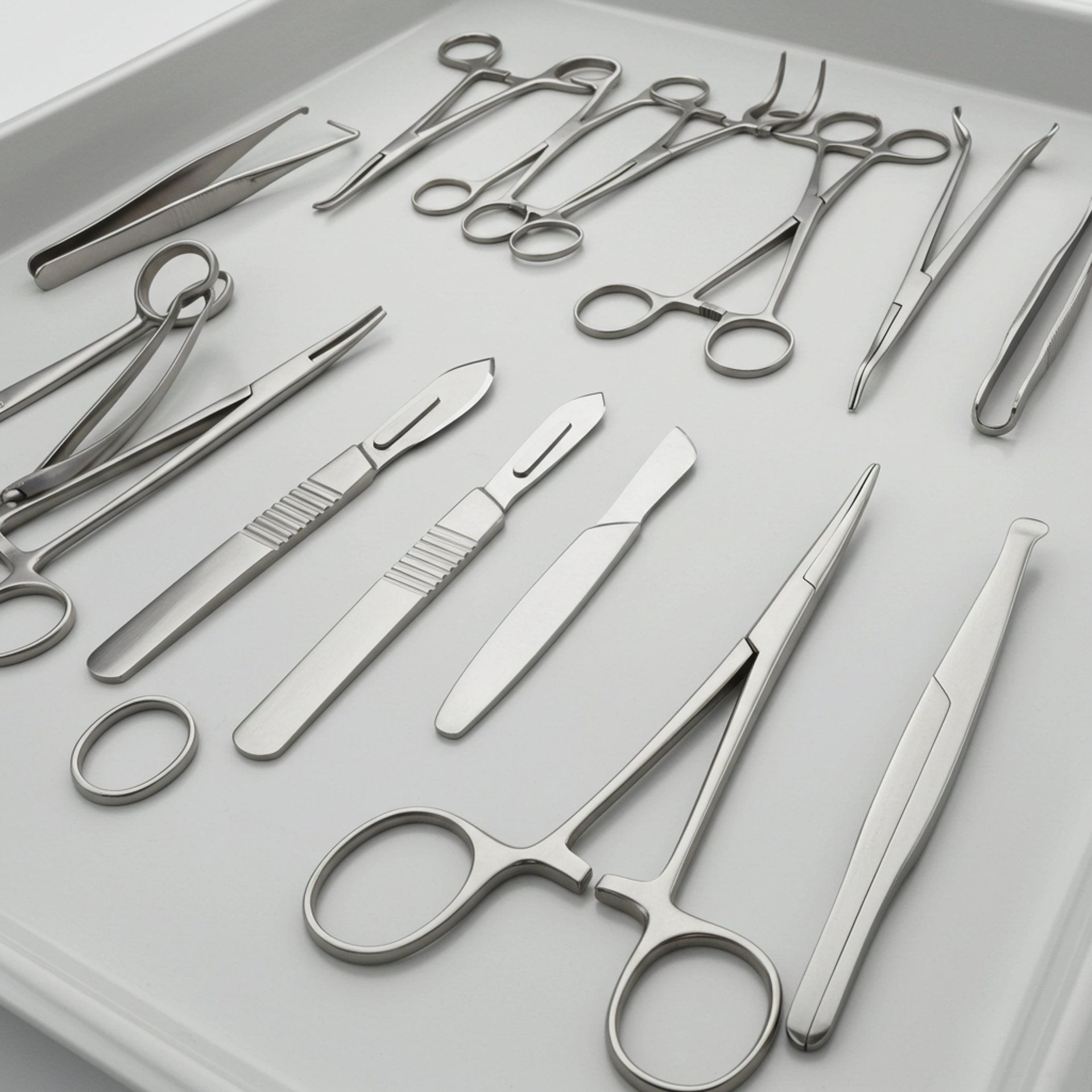
Quality Control
Zestlet Surgical | Quality Control
Commitment to Quality
At Zestlet Surgical, we are dedicated to delivering the highest quality surgical instruments and services to healthcare professionals worldwide. We foster an environment where innovation, learning, and continuous improvement are at the core of our culture. By empowering talented individuals and encouraging great ideas, we aim to set new benchmarks in the surgical manufacturing industry. Our mission is simple: to be better than yesterday, every single day.
We believe in uncompromised quality, exceeding customer expectations, and promoting a strong quality culture that drives both employee growth and customer satisfaction.
Our Global Quality Standards & Certifications
Zestlet Surgical proudly adheres to internationally recognized quality systems and has achieved the following certifications and regulatory compliance:
European Authorized Representative (EC REP) Certificate
EUDAMED SRN under EU Medical Device Regulation (EU MDR 2017/745)
QMS compliant with EU Medical Device Directive (EU MDD 93/42/EEC)
QMS according to cGMP standards by the U.S. FDA
ISO 9001 & ISO 13485 Certified Quality Management Systems
Manufacturing facilities certified with ISO 14001 & ISO 45001 standards


All surgical instruments manufactured by Zestlet Surgical are crafted using AISI grade 304 and 410/420 stainless steel, ensuring exceptional durability, corrosion resistance, and biocompatibility. Our products fully comply with internationally recognized quality standards, including ISO 9001, ISO 13485, CE Mark certification, and are FDA-approved.
To maintain the highest levels of trust and safety, we adhere strictly to global regulatory requirements and hold the following certifications and standards:
ISO 7151, ISO 7153-1, ISO 11135, ISO 13402, ISO 17664, ISO 17664-1, ISO 21850-1, ISO 14971, ISO 15223-1, ISO 20417, ISO 11607-1, ASTM A380-06, ASTM F899-20, and REACH Regulation (EU).
At Zestlet Surgical, quality is never compromised—every instrument we produce is designed to meet and exceed the most rigorous international standards.
Capability
At Zestlet Surgical, we combine a personalized approach with large-scale production capabilities. Through continuous in-house product development and strategic innovation, we have built a diverse portfolio of surgical instruments and medical solutions. Our offerings include both standard instruments and fully customized solutions, tailored to meet the unique needs of healthcare professionals and organizations worldwide.
Critical Dimensions
Each instrument undergoes rigorous quality assurance testing to guarantee flawless performance during critical surgical procedures. Precision and reliability are at the heart of everything we manufacture.
Surface Inspection
Every instrument is carefully inspected for material integrity and surface perfection. We ensure a smooth satin finish, completely free of defects, excess lubricants, or foreign substances, maintaining the highest hygienic standards.
Dimensions Verification
Critical dimensions are meticulously verified using calipers, micrometers, and specialized gauges, cross-checked against technical drawings to ensure pattern accuracy and consistency in every product.
Manufacturer & Exporter of
Dental and Surgical Instruments, Beauty Instruments, Diagnostic Devices, Hollowware and Hospital Furniture
Precision
Email:sales@zestletsurgical.com
Phone: +923294372020
© 2025. All rights reserved.
| Corporate
| Commitment
| Sustainability
| Production
| Contact US
Address: Chowk, opp. ZestletSurgical Factory, Kotli Behram, Sialkot, Punjab 51310
